One of the great things about working with aphids is that it gave me the chance to go back to my childhood entomological roots of playing with ants. Most gardeners have had the experience when cruelly* running their finger and thumb down an aphid covered plant stem of finding their hand suddenly covered with ants. As someone who has a very relaxed approach to aphids, I find the presence of ants on a plant a handy way of finding aphids, although sometimes the ants are there because of extra-floral nectaries. So what exactly is going on when you find ants and aphids together?
It has long been known that some aphids are farmed or tended by some ant species. According to Jones (1927) Goedart** was the first to describe the relationship scientifically (Goedart & Lister, 1685) and by the latter half of the 19th Century you can find illustrations such as the one below that appeared in Van Bruyssel’s fantastic foray into early science-communication.
An ant dairy maid coming to milk her aphids – their siphunculi and anuses are just visible if you look closely: cleverly made to look like cow heads (From Van Bruyssel, 1870)
The ant-aphid association is usually defined as a mutualism as the two species exist in a relationship in which each individual benefits from the activity of the other. Just to confuse people however, the association is also sometimes termed trophobiosis*** (e.g. Oliver et al., 2008) which is a more symbiotic relationship.
The degree of dependence of the aphid on the ants varies from species to species. Some aphids, especially those that live underground on plant roots, are unable to survive without their ant attendants (Pontin, 1978). Pontin (1960) also reports seeing Lasius flavus workers licking aphid eggs which he suggests stops them from going mouldy as the licking removes fungal spores. He also noted that those eggs that were not cared for in this way did not hatch. Other aphids have a more facultative relationship, and are able to survive quite successfully without the help of their friendly neighbourhood ants.
We tend to think of aphids as soft squidgy defenceless things that are easy to squash. To other insects however, they present a bit more of a challenge. Aphids have structural and behavioural defences to keep them safe in the dangerous world of bug eat bug. Alarm pheromones and dropping behaviour are commonly used by aphids to avoid meeting predators face to face (Dixon, 1958a). Aphis also have a number of physical defences. Their spihunculi (cornicles) can produce a quickly hardening wax to gum up ladybird jaws (Dixon, 1958b). Other aphid species cover themselves with dense waxy coats that make them less palatable or accessible to natural enemies (Mueller et al., 1992). Other aphids have thick skins (heavily sclerotized) and what entomologists term saltatorial leg modification; long legs to you and me, and so able to give a ladybird or other opportunistic insect predator a good kicking (Villagra et al., 2002). These characteristics, which are all costly, are reduced or absent in aphids that are frequently associated with ants (Way, 1963) as presumably with ant bodyguards in attendance, there is no need for the aphids to invest in extra anti-predator defences.
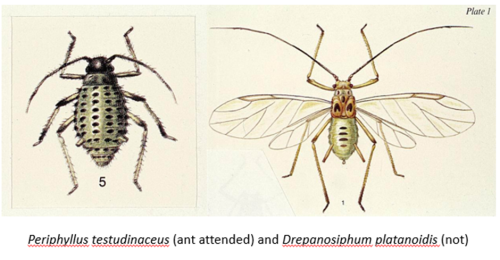
Note also the shortened siphunculi in Periphyllus testudinaceus and the hairier bottom, when compared with the leggy, and arguably, prettier Drepanosihpum platanoidis.
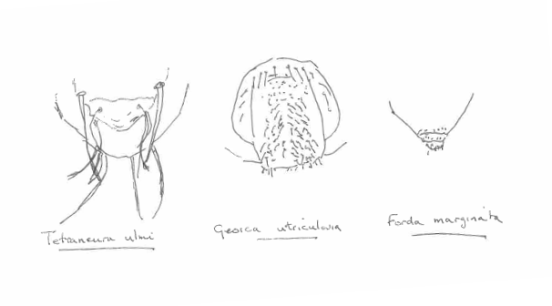
Apart from reducing their defensive armoury, those aphids that are obligately ant attended have a specially adapted rear end, essentially a hairy bottom. This is more scientifically known as the trophobiotic organ. The trophobiotic organ is an enlarged anal plate surrounded by special hairs that acts as a collection and storage device that allows the aphid to accumulate honeydew ready for the ants to remove at their leisure.
Three different trophobiotic organs, some hairier than others – after Heie (1980)
A real live view of the “trophobiotic organ” of Tetraneura ulmi (from the fantastic Influential Points website – http://influentialpoints.com/Images/Tetraneura_ulmi_aptera_on_grass_roots_c2015-09-04_14-53-13ew.jpg
Non-ant attended aphids without the trophobiotic organ, deposit their honeydew directly on to the leaf surface or on the ground, or if you are unlucky enough to park under an aphid infested tree, on to your car 🙂 Ants lick and collect sycamore aphid, Drepanosiphum platanoidis honeydew from leaves, but not directly from the aphids, which they do do from the maple aphid, Periphyllus testudinaceus, which also lives on sycamore trees P. testudinaceus (Pontin, 1958).
So what’s in it for the ants? Why should they bother looking after aphids, even in some cases, keeping aphid eggs in their nests over the winter (Pontin, 1960)? The obvious answer is the honeydew that the aphids produce as a by-product of feeding on phloem sap. The amount of material that an aphid can remove from a plant is quite astounding. A large willow aphid (Tuberolacnhus salignus) adult can sucks up the equivalent of 4 mg sucrose per day Mittler (1958) , which is equivalent to the photosynthetic product of one to two leaves per day. Admittedly, they are large aphids and not ant attended****, but even an aphid half their size passes a lot of plant sap through their digestive systems. Honeydew is not just sugar but is a mixture of free amino acids and amides, proteins, mineral and B-vitamins, so all in all, quite a useful food source for the ants (Way, 1963). All aphids produce honeydew but not all aphids are ant attended and as I pointed out earlier, not all ants attend aphids. Our research suggests that 41% of ant genera have trophobiotic species, but these are not equally distributed among ant families. Some ant sub-families, for example the Fomicinae, specilaise in ant attendance, whereas in other ant families such as the Ecitoninae, aphids are used only as prey and the honeydew is gathered from plant and ground surfaces (Oliver et al., 2008). The ant species that are most likely to develop mutualistic relationship with aphids appear to be those that live in trees, have large colonies, are able to exploit disturbed habitats and are dominant or invasive species (Oliver et al., 2008).
Those ants that do tend aphids don’t just protect them from predators and other natural enemies. They want to maximise the return for their investment. The black bean aphid, Aphis fabae, which is often tended by Lasius niger, has its tendency to produced forms reduced by the ants, thus making sure that the aphids are around longer to provide food for them (El-Ziady & Kennedy, 1956). The ant Lasius fuliginosus transports young Stomaphis quercus aphids to parts of the tree with the best honeydew production (Goidanich, 1959) and Lasius niger goes one step further, moving individuals of the aphid Pterocomma salicis, to better quality willow trees (Collins & Leather, 2002). Lasius niger seems to have a propensity for moving bugs about, they have also been seen moving coccids from dying clover roots to nearby living ones (Hough, 1922).
In the mid-1970s John Whittaker and his student, Gary Skinner, set up a study to examine the interactions between the wood ant, Formica rufa and the various insect herbivores feeding on the sycamore trees in Cringlebarrow Wood, Lancashire. They excluded some ants from some of the aphid infested branches and allowed them access to others on the same trees and also looked at trees that were foraged by ants and those that weren’t. They found that F. rufa was a heavy predator of the sycamore aphid, D. platanoidis, but tended the maple aphid, P. testudinaceus (a novel observation for that particular ant-aphid interaction). Ant excluded colonies of P. testudinaceus decreased, whereas D. platanoidis did not, but on those branches where ants were able to access the aphids, the reverse pattern was seen (Skinner & Whittaker, 1981).
The presence of thriving aphid colonies in the neighbourhood of ant nests and in some cases aphid colonies only exist where there are ant nests nearby (Hopkins & Thacker, 1999), has made some people wonder if aphids actively look for ant partners (Fischer et al., 2015). There is, however, no evidence that aphids look for ant partners, rather the fact that wing production is reduced in the presence of tending ants, means that aphid colonies can accumulate around and close to ant nests (Fischer et al., 2015a).
That doesn’t mean that the aphids only rely on honeydew production to guarantee the presence of their ant bodyguards. The aphid Stomaphis yanonis, which like other
Stomaphis aceris, also ant attended. Imagine trying to drag that mouth part out of a tree trunk quickly 🙂
Stomaphis species, has giant mouthparts, and so needs plenty of time to remove its mouthparts safely definitely needs ant protection to cover its back when involved in the delicate operation of stylet unplugging. In this case, it turns out that the aphids smell like that ants, they have cuticular hydrocarbons that resemble those of their ant protector Lasius fuji and thus encourages the ants to treat them as their own (Endo & Itino (2013). Earlier work on the ant-attended tree-dwelling aphids, Lachnus tropicalis and Myzocallis kuricola, in Japan showed that the ant Lasius niger preyed on aphids that had not been attended by nest mates, but tended those that had been previously tended (Sakata 1994). This too would indicate the presence of some sort of chemical marker or brand.
To add support to this, just over twenty years ago (1996), I supervised an undergraduate student Arran Frood*****. He worked with the maple aphid, and the ants L. niger and L. fulginosus. Aphids on ant-attended sycamore trees were washed with diluted acetone or water. Those that had been washed with acetone were predated more than unwashed aphids suggesting that It was like washing off the colony specific pheromone marker. In support of this hypothesis, Arran found that predation would also increase if he swapped a twig full of aphids between colonies, but not from one part of the colony to another. It also worked between the two ant species, Lasius niger and L. fuliginosus, so it seems like the ants have a colony specific marker on their aphids. We should really have written this up for publication.
Although aphids do not actively seek ant partners, they may compete with each other to retain the services of their ant bodyguards by producing more honeydew (Addicott, 1978). There is evidence that ants make their decisions of whether to predate or tend aphids by monitoring honeydew production and choose to prey on aphids in colonies that produce less honeydew (Sakata, 1995). Recent work has also shown that the honeydew of the black bean aphid, Aphis fabae is often colonised by the bacterium Staphylococcus xylosus. Honeydew so infected produces a bouquet of volatile compounds that are attractive to the ant L. niger thus increasing the cahnces of the aphids being ant-attended (Fischer et al., 2015b). This adds yet another layer of complexity to the already complicated mutualistic life style that aphids have adopted.
And finally, you may remember me writing about the wonderful colour variations seen in some aphid species and how this could be modified by their symbionts. In another twist, it seems that ants may have a say in this too, albeit at a colony level rather than at the clonal level. The improbably named Mugwort aphid, Macrosiphoniella yomogicola which is obligately ant-attended by the ant L. japonicus, is found in colonies that are typically 65% green 35% red (Watanabe et al. 2016). The question Watanabe and his colleagues asked is why do ants like this colour balance? One possibility is that red and green aphids have slightly different effects on the mugwort plants where they feed. Though green aphids produce more honeydew, red aphids seem to prevent the mugwort from flowering. Given that aphid colonies on a flowering mugwort go extinct, ants looking to maintain an aphid herd for more than a year might see an advantage to keeping reds around to guarantee a long-term food supply from their green sisters.
Aren’t insects wonderful?
References
Addicott, J.F. (1978) Competition for mutualists: aphids and ants. Canadian Journal of Zoology, 56, 2093-2096.
Carroll, C.R. & Janzen, D.H. (1973) Ecology of foraging by ants. Annual Review of Ecology & Systematics, 4, 231-257
Collins, C.M. & Leather, S.R. (2002) Ant-mediated dispersal of the black willow aphid Pterocomma salicis L.; does the ant Lasius niger L. judge aphid-host quality? Ecological Entomology, 27, 238-241.
Dixon, A.F.G. (1958a) The escape responses shown by certain aphids to the presence of the coccinellid Adalia decempunctata (L.). Transactions of the Royal Entomological Society London, 110, 319-334.
Dixon, A.F.G. (1958b) The protective function of the siphunculi of the nettle aphid, Microlophium evansi (Theob.). Entomologist’s Monthly Magazine, 94, 8.
El-Ziady, S. & Kenendy, J.S. (1956) Beneficial effects of the common garden ant, Lasius niger L., on the black bean aphid, Aphis fabae Scopoli. Proceedings of the Royal Entomological Society London (A), 31, 61-65
Endo, S. & Itino, T. (2012) The aphid-tending ant Lasius fuji exhibits reduced aggression toward aphids marked with ant cuticular hydrocarbons. Research on Population Ecology, 54, 405-410.
Endo, S. & Itino, T. (2013) Myrmecophilus aphids produce cuticular hydrocarbons that resemble those of their tending ants. Population Ecology, 55, 27-34.
Fischer, C.Y., Vanderplanck, M., Lognay, G.C., Detrain, C. & Verheggen, F.J. (2015a) Do aphids actively search for ant partner? Insect Science, 22, 283-288.
Fischer, C.Y., Lognay, G.C., Detrain, C., Heil, M., Sabri, A., Thonart, P., Haubruge, E., & Verheggen, F.J. (2015) Bacteria may enhance species-association in an ant-aphid mutualistic relationship. Chemoecology, 25, 223-232.
Goidanich, A. (1959) Le migrazioni coatte mirmecogene dello Stomaphis quercus Linnaeus, afido olociciclio monoico omotopo. Bollettino dell’Istituto di Entomologia della Università degli Studi di Bologna, 23, 93-131.
Goedart, J. & Lister, M. (1685) De Insectis, in Methodum Redactus; cum Notularum Additione. [Metamorphosis Naturalis] Smith, London.
Heie, O. (1980) The Aphdioidea (Hemiptera) of Fennoscandia and Denmark. 1. Fauna Entomologica Scandinavica 9.Scandinavian Science Press, Klampenborg, Denmark.
Hough, W.S (1922) Observations on two mealy bugs Trionymus tritolii Forbes and Pseudococcus maritimus Ehrh. Entomologist’s News, 33, 1 7 1-76.
Hopkins, G.W. & Thacker, J.I. (1999) Ants and habitat specificity in aphids. Journal of Insect Conservation, 3, 25-31.
Jones, C.R. (1927) Ants and Their Relation to Aphids. PhD Thesis, Iowa State College, USA.
Mittler, T.E. (1958a) Studies on the feeding and nutrition of Tuberolachnus salignus (Gmelin) (Homoptera, Aphididae). II. The nitrogen and sugar composition of ingested phloem sap and excreted honeydew. Journal of Experimental Biology, 35, 74-84.
Mueller, T.F., Blommers, L.H.M. & Mols, P.J.M. (1992) Woolly apple aphid (Eriosoma lanigerum Hausm., Hom., Aphidae) parasitism by Aphelinus mali Hal. (Hym., Aphelinidae) in relation to host stage and host colony size, shape and location. Journal of Applied Entomology, 114, 143-154.
Oliver, T.H., Leather, S.R. & Cook, J.M. (2008) Macroevolutionary patterns in the origin of mutualisms, Journal of Evolutionary Biology, 21, 1597-1608.
Pontin, A.J. (1958) A preliminary note on the eating of aphids by ants of the genus Lasius. Entomologist’s Monthly Magazine, 94, 9-11.
Pontin, A.J. (1960) Some records of predators and parasites adapted to attack aphids attended by ants. Entomologist’s Monthly Magazine, 95, 154-155.
Pontin, A.J. (1960) Observations on the keeping of aphid eggs by ants of the genus Lasius. Entomologist’s Monthly Magazine, 96, 198-199.
Pontin, A.J. (1978) The numbers and distributions of subterranean aphids and their exploitation by the ant Lasius flavus (Fabr.). Ecological Entomology, 3, 203-207.
Sakata, H. (1994) How an ant decides to prey on or to attend aphids. Research on Population Ecology, 36, 45-51.
Sakata, H. (1995) Density-dependent predation of the ant Lasius niger (Hymenoptera: Formicidae) on two attendant aphids Lachnus tropicalis and Myzocallis kuricola (Homoptera: Aphidae). Research on Population Ecology, 37, 159-164.
Skinner, G.J. & Whittaker, J.B. (1981) An Experimental investigation of inter-relationships between the wood-ant (Formica rufa) and some tree-canopy herbivores. Journal of Applied Ecology, 50, 313-326.
Stadler, B. & Dixon, A.F.G. (1999) Ant attendance in aphids: why different degrees of myrmecophily? Ecological Entomology, 24, 363-369.
Van Bruyssel, E. (1870) The Population of an Old Pear Tree. MacMillan & Co, London
Vilagra, C.A., Ramirez, C.C. & Niemeyer, H.M. (2002) Antipredator responses of aphids to parasitoids change as a function of aphid physiological state. Animal Behaviour, 64, 677-683.
Watanabe, S., Murakami, T., Yoshimura, J. & Hasegawa, E. (2016) Color piolymorphism in an aphid is maintained by attending ants. Science Advances, 2, e1600606
Way, M.J. (1963) Mutualism between ants and honeydew-producing Homoptera. Annual Review of Entomology, 3, 307-344.
*in my opinion at any rate 🙂
**I have had to take this on faith as have not been able to get hold of the original reference and read it myself
***Trophobiosis is a symbiotic association between organisms where food is obtained or provided. The provider of food in the association is referred to as a trophobiont. The name is derived from the Greek τροφή trophē, meaning “nourishment” and -βίωσις -biosis which is short for the English symbiosis
****Perhaps they are too big for ants to mess with? They are, however, very often surrounded by Vespid wasps who do appreciate the huge amount of honeydew deposited on the willow leaves and stems.
***** He must have enjoyed it because he also did his MSc project with me the following year 🙂
Post script
I began this post with an illustration from Van Bruyssel. I finish it with this illustration from another early attempt to get children interested in entomology. Unfortunately in this case the ant attended aphids are the very opposite of what they should look like and he further compounds his error by telling his youthful audience that the aphids milk the aphids via their siphunculi 😦
The very opposite of what an ant-attend aphid looks like – from Half hours in the tiny world; wonders of insect life by C.F. Holder (1905)