In 1981 I spent a lot of time trudging through snow, cross-country skiing and snow-shoeing my way across the snowy wastes of Finland to snip twigs off bird cherry trees. This was part of my post-doc which was to develop a forecasting system for the bird cherry-oat aphid, Rhopalosiphum padi. On returning to the lab I then spent many a happy hour counting how many aphid eggs were nestled in between the buds and the stem on each twig. It was while doing this that I noticed that some of the twigs were infested with the overwintering larval shields of the bird cherry ermine moth, Yponomeuta evonymellus. Of course I then started counting them as well 🙂 I noticed that trees with lots of aphid eggs didn’t have very many larval shields and I wondered why. Some later observations from marked trees in Scotland appeared to provide evidence that the aphids and the moths tended to either prefer different trees or perhaps excluded each other.
Negative correlation between moths and aphids – more moths equals fewer aphids and vice versa
Based on these data I hypothesised that the two insects were indirectly competing for resources by altering plant chemistry and/or architecture thus making the trees less or more suitable for egg laying in the autumn (Leather, 1988). I tested this experimentally when I was working for the Forestry Commission in Scotland using potted bird cherry trees that I defoliated to a lesser or greater extent to see if I could induce changes in foliar quality and tree growth rates that might influence subsequent colonisation by the aphids and moths. As predicted, those trees that had been defoliated, albeit by me and not by moth larvae, were less attractive to aphids in the autumn (Leather, 1993). These effects were still apparent five years after the beginning of the experiment (Leather, 1995) when I had to desert my trees as I moved to a new position at Imperial College’s Silwood Park campus.
Given that apart from the location, the SE of England, this was my idea of a dream job for life (colleagues at the time included John Lawton, Mike Hassell, Bob May, Stuart McNeill, Mike Way, Brad Hawkins, Shahid Naeem, Mike Hochberg, Chris Thomas to name but a few), I decided to start up two long-term projects to see me through the next 30 years, one observational (my 52 sycamore tree project), the other experimental, a follow up to my bird cherry defoliation experiment.
I went for a simplified design of my earlier experiments, just two defoliation regimes, one to mimic aphid infestation (50%), the other to mimic bird cherry ermine moth defoliation (100%) and of course a non-defoliated control. I also planted the trees in the ground to better simulate reality. Using potted plants is always a little suspect and I figured that I would need to do rather a lot of re-potting over the next 30 years 🙂
The grand plan!
I sourced my trees from a Forestry Commission nursery thinking that as the national organisation responsible for tree planting in the UK I could trust the provenance of the trees. Things didn’t go well from the start. Having planted my trees in autumn 1992 and established the treatments in the spring of 1993 I discovered that my bird cherry, rather than being from a native provenance (seed origin) were originally from Serbia! Hmm 🙂 It was too late to start again, so I decided to carry on. After all, bird cherry although widely planted in the SE, has a native distribution somewhat further north and west, which meant I was already operating close to the edge of ‘real life’, so what did an extra 1600 kilometres matter?
The mainly ‘natural’ distribution of bird cherry (left, Leather, 1996) and the current distribution including ‘introduced’ trees https://www.brc.ac.uk/plantatlas/index.php?q=plant/prunus-padus
Next, I discovered that my fence was neither rabbit nor deer proof. I almost gave up at this point, but having invested a lot of time and energy in setting up the plot I once again decided to carry on. On the plus side, the trees most heavily defoliated and bitten back were mainly from the 100% defoliation treatment, but did give me some negative growth rates in that year.
My original plan was to record height (annually), bird cherry egg numbers (every December), bird cherry ermine moth larval shields (annually), bud burst and leaf expansion once a week, leaf-fall (annually), and once a month, defoliation rates in two ways, number of damaged leaves and an overall estimation of percentage defoliation. This was a personal project, so no grant funding and no funding for field assistants. It soon became clear, especially when my teaching load grew, as Imperial started replacing whole organism biologists with theoretical and molecular biologists, and I was drafted in to take on more and more of the whole organism lecturing, that I would not be able to keep both of my long term projects going with the same intensity. Given the ‘problems’, associated with the bird cherry project, I decided that I would ditch some of my sampling, bud burst was scored on 21st March every year and defoliation only measured once, in late summer and egg sampling and height recording came to a halt once the trees grew above me (2005)! This allowed me to carry on the sycamore project as originally intended*.
I kept an eye on the trees until I left Silwood Park in 2012, but by 2006 I was only monitoring bud burst and leaf fall feeling that this might be useful for showing changes in phenology in our ever-warming world. One regret as I wandered between the then sizeable trees in the autumn of 2012 was that I had not taken a before and after photograph of the plots. All I have are two poor quality photos, one from 2006, the other from 2012.
The Sixty Tree site April 2006.
The Sixty Tree site April 2010 with a very obvious browse line
So, after all the investment in time, and I guess to a certain extent money (the trees and the failed fencing, which both came out of my meagre start-up funding**), did anything worthwhile come out of the study?
The mean number of Rhopalosiphum padi eggs per 100 buds in relation to defoliation treatment
As a long-time fan of aphid overwintering it was pleasing to see that there was a significant difference not only between years (F= 8.9, d.f. = 9/29, P <0.001), but also between treatments with the trees in the control treatment having significantly more eggs laid on them than the 100% defoliation treatment (F= 9.9, d.f. = 2/ 29, P <0.001 with overall means of 1.62, 1.22 and 0.65 eggs/100 buds). This also fitted in with the hypothesis that trees that are defoliated by chewing herbivores become less suitable for aphids (Leather, 1988). I must admit that this was a huge surprise to me as I had thought that as all the trees were attacked by deer the year after the experimental treatments they would all respond similarly, which is why I almost gave up the experiment back in 1994.
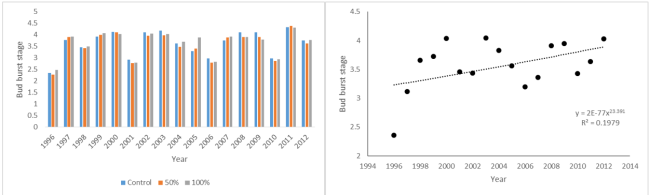
Bud burst stage of Prunus padus at Silwood Park on March 21st 1996-2012; by treatment and combined
When it came to budburst there was no treatment effect, but there was a significant trend to earlier budburst as the trees became older which was strongly correlated with warmer springs, although as far as spring temperatures were concerned there was no significant increase with year.
Mean spring temperature (Silwood Park) 1993-2012 and relationship between mean spring temperature and bud bust stage on 21st March.
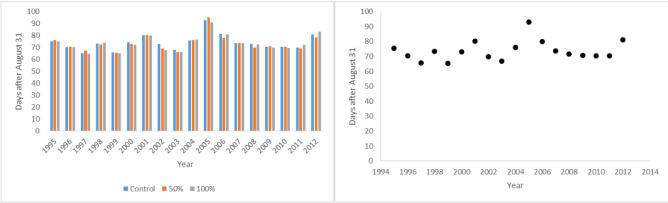
Mean date of final leaf fall of Prunus padus at Silwood Park 1995-2012; by treatment and combined
At the other end of the year, there was a significant difference between date of final leaf fall between years but no significant difference between treatments. In retrospect I should have adopted another criterion. My date for final leaf fall was when the last leaf fell from the tree. Those of you who have watched leaves falling from trees will know that there are always a few who are reluctant to make that drop to the ground to become part of the recycling process. Even though they are very obviously dead, they hang there until finally dislodged by the wind. I should really have used a measure such as last leaf with any pigment remaining. I am sure that if I could be bothered to hunt down the wind speed data I would find that some sort of correlation.
Mean height (cm) of Prunus padus trees at Silwood Park 1993-2005 and Diameter at Breast Height (DBH) (cm) at the end of 2012
Except for the year after the deer attack, the trees, as expected, grew taller year by year. There was however, no significant difference between heights reached by 2005 or in DBH at the end of 2012 despite what looked like a widening gap between treatments.
Defoliation scores of Prunus padus at Silwood Park 1993-2004; % leaves damaged and overall defoliation estimates
My original hypothesis that trees that were heavily defoliated at the start of their life would be more susceptible to chewing insects in later life, was not supported. There was no significant difference between treatments, although, not surprisingly, there was a significant difference between years. Average defoliation as has been reported for other locations was about 10% (Kozlov et al., 2015; Lim et al., 2015).
Number of Prunus padus trees with severe deer damage
That said, when I looked at the severity of deer attack, there was no effect of year but there was a significant effect of treatment, those trees that had been 100% defoliated in 1993 being most attractive to deer. In addition, 20% of those trees were dead by 2012 whereas no tree deaths occurred for the control and less severely defoliated treatments.
I confess to being somewhat surprised to find as many significant results as I did from this simple analysis and was momentarily tempted to do a more formal analysis and submit it to a journal. Given, however, the number of confounding factors, I am pretty certain that I would be looking at an amateur natural history journal with very limited visibility. Publishing it on my blog will almost certainly get it seen by many more people, and who knows may inspire someone to do something similar but better.
The other reason that I can’t be bothered to do a more formal analysis is that my earlier work on which this experiment was based has not really hit the big time, the four papers in question only accruing 30 cites between them. Hardly earth shattering despite me thinking that it was a pretty cool idea; insects from different feeding guilds competing by changing the architecture and or chemsitry of their host plant. Oh well. Did anything come out of my confounded experiment or was it a total waste of time? The only thing published from the Sixty Trees was a result of a totally fortuitous encounter with Marco Archetti and his fascination with autumn colours (Archetti & Leather, 2005), the story of which I have related in a previous post, and which has, in marked contrast to the other papers, had much greater success in the citation stakes 🙂
And finally, if anyone does want to play with the data, I am very happy to give you access to the files.
References
Archetti, M. & Leather, S.R. (2005) A test of the coevolution theory of autumn colours: colour preference of Rhopalosiphum padi on Prunus padus. Oikos, 110, 339-343. 50 cites
Kozlov, M.V., Lanta, V., Zverev, V., & Zvereva, E.L. (2015) Global patterns in background losses of woody plant foliage to insects. Global Ecology & Biogeography, 24, 1126-1135.
Leather, S.R. (1985) Does the bird cherry have its ‘fair share’ of insect pests ? An appraisal of the species-area relationships of the phytophagous insects associated with British Prunus species. Ecological Entomology, 10, 43-56. 14 cites
Leather, S.R. (1988) Consumers and plant fitness: coevolution or competition ? Oikos, 53, 285-288. 10 cites
Leather, S.R. (1993) Early season defoliation of bird cherry influences autumn colonization by the bird cherry aphid, Rhopalosiphum padi. Oikos, 66, 43-47. 11 cites
Leather, S.R. (1995) Medium term effects of early season defoliation on the colonisation of bird cherry (Prunus padus L.). European Journal of Entomology, 92, 623-631. 4 cites
Leather, S.R. (1996) Biological flora of the British Isles Prunus padus L. Journal of Ecology, 84, 125-132. 14 cites
Lim, J.Y., Fine, P.V.A., & Mittelbach, G.G. (2015) Assessing the latitudinal gradient in herbivory. Global Ecology & Biogeography, 24, 1106-1112.
*which you will be pleased to know, is being analysed as part of Vicki Senior’s PhD project, based at the University of Sheffield.
**£10 000 which even in 1992 was not overly-generous.